
Index (Alphabetical order)
First, you should totally download the PDF (see left) for a thorough explanation of the tests from "Guidelines for quick application of biochemical tests to identify unknown bacteria" from ResearchGate.net . I have summarized many of these tests from this paper. Seriously, download them!
The Basics of Biochemical Testing for Bacteria Identification
General Info
-
Ensure the correct site, container and volume is processed for each specimen. In general, the more volume of fluid, the better yield.
-
Optimal specimen for diagnosis of infective bacterial infection in a blood culture bottle= 3 peripheral blood samples (20mL/set) collected every 8 hours over a 24 hour period.
-
Testing Process
1. Perform a Gram stain and examine the staining & morphological characteristics.
-
See my posts on The Basics of Gram Staining & Classifying Bacteria by Morphology for more details. We will discuss these thoroughly later.
-
Can classify organisms as gram positive or gram negative organisms based on characteristic differences in the cell walls of the organisms.
-
Can broadly classify by morphology into cocci (round/spherical) or bacilli (rod/cylindrical) organisms.
-
See detailed posts on the laboratory workup of:
2. Determine the organism's oxygen requirements (Aerobic vs. Anaerobic Testing) for bacteria detection.
-
See full post on Aerobic vs. Anaerobic Organisms for more details on the classification of bacteria based on the oxygen requirements.
-
Culture methods & Growth medias (agars, broths, medias)
-
Non culture methods:
-
Antigen detection (fluorescent Ab, latex agglutination)
-
Nucleic Acid detection (probes, nucleic acid amplification)
-
3. For bacteria unable to form spores and not easily categorized via gram staining methods, other stains can be used:
-
Acridine Orange
-
Fluorescent antibodies
-
Methylene blue
-
Modified Kinyoun
-
Wayson stain
4. Biochemical Tests can be used to distinguish between the various bacteria based on the presence or absence of various enzymes.
5. Antibiotic Susceptibility Testing can be used to help distinguish between various organisms and to help guide therapy to determine whether an organism is susceptible or resistant to killing with a particular antibiotic.
Laboratory Testing for Bacteria
(Alphabetical Order)
Acid-fast bacteria contain a lipid-rich cell wall with mycolic acid that is relatively impermeable to most basic dyes unless they are combined with phenol. The primary stain used is Carbolfuchsin, a lipid soluble stain containing phenol which (with heat application) allows the stain to penetrate the cell wall. Once stained, the cells resist decolorization with acidified organic solvents and are therefore called "acid-fast".
See detailed post about acid-fast staining organisms.

ACID FAST STAINING
LOWEST
oxygen
requirements
HIGHEST
oxygen
requirements

AEROBE VS. ANAEROBE TESTING
-
Aerobic and anaerobic bacteria can be identified by growing them in tubes of broth containing Thioglycollate which reacts with oxygen and consumes it.
-
In a tube of Thioglycollate broth, the oxygen concentration is decreased with increasing tube depth.
-
The position of bacterial colonies determines whether they are aerobic or anaerobic.
Growth at High O2
Growth at Low O2
Growth in presence and absence of O2
OBLIGATE aerobES
-
Require oxygen for growth.
-
These organisms use glycolysis, the Krebs TCA cycle and electron transport chain with oxygen as the final electron acceptor.
-
Possess all 3 enzymes:
-
Catalase
-
Peroxidase
-
Superoxide dismutase
-
facultative aerobES
-
Prefers oxygen for growth, but can grow without oxygen by using fermentation for energy.
-
Possess 2 enzymes:
-
Catalase
-
Superoxide dismutase
-
MICRO-
AEROPHILIC
-
Also called "Aero-tolerant" anaerobes
-
Requires reduced oxygen concentration for growth.
-
Use fermentation for energy
-
Have no electron transport system
-
Have 1 enzyme:
-
Superoxide dismutase
-
OBLIGATE ANaerobES
-
CANNOT grow in the presence of oxygen.
-
Lacks all 3 enzymes required for oxygen breakdown.
-
See full post on the Classification of Aerobes vs. Anaerobes by their Gram Staining patterns
SENSITIVE
RESISTANT

ANTIBIOTIC SENSITIVITY TESTING (Agar diffusion test)

ANTIGEN DETECTION FOR BACTERIA IDENTIFICATION
Certain antigens found the surface of bacteria can be targeted and testing methods have been developed to detect them.

Fluorescent antibodies
-
Bordetella
-
Chlamydia
-
Legionella
Latex agglutination
-
Detects:
-
H. influenzae
-
N. meningitidis
-
S. agalactiae
-
S. pneumoniae
-
-
Used in the past for detection of CSF pathogens in partially treated meningitis.
-
Not routinely used nowadays.
Stool testing available:
-
Helicobacter pylori
Urine antigen testing available:
-
Legionella
-
S. pneumoniae



Catalase: an enzyme that catalyzes the release of oxygen from hydrogen peroxide (H2O2)
CATALASE TESTING




CATALASE NEGATIVE:
No bubbles are formed
CATALASE POSITIVE:
Bubbles are formed




COAGULASE TESTING
COAGULASE NEGATIVE:
No clot formed on slide (left) or tube (right) tests
COAGULASE POSITIVE:
Clots/clumps formed on slide (left) or tube (right) tests
CULTURES (Plating media and broths used to grow bacteria)
GENERAL INFORMATION
GROWTH CONDITIONS (See post on Aerobic vs. Anaerobic organisms for more details)
-
Aerobic- utilize glucose in the presence of oxygen
-
Facultative aerobes- utilize glucose with or without oxygen (most bacteria are facultative- Enterobacteriacae, Staphylococcus)
-
-
Anaerobic- will NOT grow in oxygen. Require CO2, H2 or N2 for growth
-
Aerotolerant- anaerobes that survive temporary exposure to oxygen (Clostridium tertium).
-
Microaerophilic- require increased CO2 & reduced O2.
-
Capnophilic- required increased CO2 to grow
-
DEFINITIONS
-
General purpose media allows the growth of most bacteria- aerobic and facultative anaerobic organisms.
-
Ex: Blood agar
-
-
Enriched media contains special nutrients that allow fastidious organisms to grow better than others.
-
Ex: Chocolate agar
-
-
Selective media (ex: Xylose lysine desoxycholate & MacConkey) contains additives that enhance the presence of desired organisms by inhibiting other organisms.
-
Ex: Xylose Lysine Desoxycholate agar, MacConkey agar
-
-
Differential media aids in the presumptive ID of organisms by causing a color change or a distinct growth pattern in the media. Also used to differentiate between the Streptococcus species due to their various hemolytic properties (alpha, beta or gamma hemolysis).
-
Ex: Hektoen or chromogenic agar, Alpha/beta/gamma hemolysis on blood agar
-
-
Specialized media is designed specifically to isolate a specific pathogen.
-
Ex: Mueller Hinton, Laked KV, BYCE (Legionella), Regan-Lowe (Bordetella)
-
Agar plates (
5% Sheep Blood Agar (Blood Agar)

-
Differential media containing a concentration of 5% mammalian blood. Also contains meat extract, tryptone, sodium chloride and agar. (General Purpose Media)
-
Used to isolate aerobic and fastidious (facultatively anaerobic) organisms and detect hemolytic activity.
BHI Broth (Brain Heart Infusion)
-
General purpose nutrient medium for the cultivation and isolation of a variety of fastidious and nonfastidious microorganisms, including bacteria, yeasts, and mold
-
Enriched with Fildes is useful for cultivating capsular strains of Haemophilus influenzae. Doesn’t have a lot of added nutrients that might get in the way of growth in the presence of Abx.

Chocolate Agar

-
Essentially, this is a blood agar plate in which the RBCs have been lysed by heating to 56 degrees C and contains special nutrients that help certain bacteria grow. (Enriched media)
-
Used for growing fastidious organisms like Neisseria & Hemophilus.
CNA Agar (Colistin, Nalidixic Acid)
-
Colistin, nalidixic acid (I)
-
Inhibits Gram negative bacteria (think N for Neg)
-
-
Allows growth of Gram positive bacteria.
-
However, most strains of S. saprophyticus and some S. aureus are inhibited.
-

MacConkey Agar

-
Bile salts, crystal violet (I), lactose, neutral red (D) (Selective media)
-
Inhibits Gram positive organisms
-
-
Enriches for Salmonella spp., Shigella spp., Yersinia spp
-
Lactose fermenters produce pink colonies
-
PEA Agar (PhenylEthyl Alcohol)
-
Penylethyl alcohol (I)
-
Inhibits Gram negative bacteria
-
Allows growth of Gram positive bacteria.

Thayer-Martin Agar

-
Composition:
-
Vancomycin to inhibit growth of Gram positive bacteria
-
Colistin to inhibit growth of Gram negative bacteria
-
Trimethoprim to inhibit growth of Proteus
-
Nystatin, Amphotericin B or Anisomycin to inhibit growth of yeast
-
-
Inhibits Gram positive bacteria and Gram negative bacilli
-
Enriches for Neisseria
Thioglycollate Broth
-
Oxygen levels are reduced via sodium thioglycolate;
-
This produces a range of oxygen levels in the media that decreases with increasing distance from the surface.
-
Highest oxygen concentration at top of tube
-
Lowest/no oxygen at bottom of tube
-
-
Allows for differentiation of aerobic, anaerobic, and facultatively anaerobic organisms based upon where they grow in the tube
-
Organisms that are able to utilize glucose in the presence of oxygen contain certain enzymes (catalse, superoxide dismutase & peroxidase) that work to detoxify the reactive oxygen species generated in the breakdown of oxygen. Some organisms lack one or all three of these enzymes and thus require reduced levels of oxygen for growth.
-
-
Used to isolate strict anaerobes
-
See post on Aerobic vs. Anaerobic organisms for more details.

XLD Agar

-
Bile salts, NaCl (I), phenol red (D), lactose, sucrose, ferric ammonium citrate for hydrogen sulfide production (D)
-
Inhibits Gram positive bacteria
-
Enriches for enteric pathogens (Salmonella spp., Shigella spp., Yersinia spp)
-
What you need to know:
-
Since clindamycin and streptogramin are among the few drugs of choice in the treatment of methicillin resistant S. aureus (MRSA) infections, knowing the resistance to these antibiotics is imperative.
-
The D test is used to study the macrolide lincosamide streptogramin resistance (MLSB), both constitutive and inducible as well as macrolide streptogramin resistance (MSB) in Staphylococcus aureus.
-
Basically, the D test is performed to determine if S. aureus is susceptible to Clindamycin.
-
-
How testing is performed:
-
Erythromycin (macrolide) and clindamycin (lincosamide derivative) discs are placed adjacent to each other over the Mueller Hinton agar medium inoculated with the test organism.
-
The test is performed in the same MHA plate in which the antibiotic sensitivity test is being done, taking into consideration that the discs are placed adjacent to each other maintaining the distance.
-
Clindamycin resistance can be induced by Erythromycin which causes the Kirby Bauer zone around the Clindamycin disc to be blunted and form a "D" if clindamycin can be induced by erythromycin to be resistant. So this is called INDUCABLE RESISTANCE.
-
D-TESTING (Clindamycin Induction Test)


D
-
Interpretation:
-
The organism susceptible to clindamycin without any flattening of the zone (D test negative) near clindamycin disc (resistant) = Macrolide streptogramin resistance (MSB)
-
Susceptible to both antibiotics = No resistance
-
Flattening of the clindamycin zone (D test positive) near the erythromycin disc (resistant) = Inducible MLSB (IMLSB)
-
The growth of the organism up to the edges of the disc = Constitutive MLSB (CMLSB) Resistance
-
Gram-staining was first described by Christian Gram in 1884. It is a differential staining technique that uses a primary stain (crystal violet) and a secondary counterstain (safranin) to distinguish between gram-positive and gram-negative bacteria based on differences in the thickness and permeability of their cell surface to the dyes (we will discuss these properties in the next lecture). Gram staining is fast and can provide immediate information regarding the presence/absence of bacteria, ultimately guiding the clinician toward the appropriate antibiotic treatment.
GRAM STAINING




See full post on Gram Staining for more details.
-
Primary stain= Crystal violet= Gram positive
-
Mordant= Gram's iodine
-
Traps the crystal violet in the peptidoglycan cell wall
-
-
Decolorizer= acetone/alcohol
-
**MOST IMPORTANT STEP**
-
This step removes stain from thin PG layer of gram-negative organisms
-
-
Secondary stain= Safranin = Gram negative
-
Could use Carbafuscin or other secondary stain in certain organisms
-
How to tell if it is a good gram stain:
-
The neutrophils should be pink.
-
If blue neutrophils, then you didn't decolorize enough.
-
If clear/washed out neutrophils, then you over-decolorized
-
-
Evaluate BAL specimen quality at 10X:
-
You want to see a lot of PMNs to ensure it is sputum and not just spit.
-
If the specimen consists of >25 squamous epithelial cells, then it is just spit. NOT acceptable for routine bacterial culture
-
Can still be sent for AFB or Legionella though
-
-
-
Evaluate bacterial morphology on oil immersion (100X) for presumptive ID of likely pathogen.
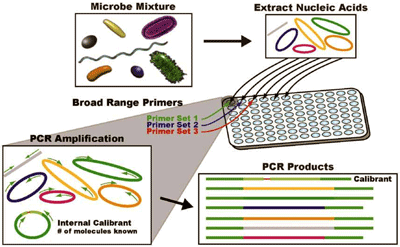
NUCLEIC ACID DETECTION
-
The identification of DNA found in bacteria is one method of detection of bacteria.
-
This utilizes specific probes that are unique to an organism and then nucleic acid amplification (PCR) can be used to determine if the organism is present.
-
Panels are available that can identify multiple organisms simultaneously.
-
-
This method is used to detect:
-
slow growing organisms
-
ones that are difficult to grow on routine culture (Chlamydia, N. gonorrhoeae, Bordetella, Bartonella)
-
uncultivable organisms (Tropheryma whippelii).
-

STAINING FOR BACTERIA
Other stains can be used to highlight various bacterial features (besides the Gram stain and Acid fast stains discussed in detail in their respective sections).
-
Acridine orange
-
Detects some bacteria in gram-stain negative blood cultures
-
Used if you clearly have bacterial growth in broth/tube, but gram stain turns out to be negative.
-
-
-
Fluorescent antibodies
-
Chlamydia trachomatis
-
-
Methylene Blue Stain
-
Good for staining PMNs in stool specimens
-
Metachromatic granules in C. diphtheriae
-
-
Modified Kinyoun
-
Nocardia
-
-
Wayson Stain
-
Bipolar staining of Yersinia pestis
-
The purpose of the starch hydrolysis test is to determine whether bacteria are able to produce the alpha-amylase enzyme by using starch as a complex hydrocarbon from glucose. Flooding the plate with iodine will turn starch blue-black. If bacteria produce amylase, starch is digested and these areas will remain clear.
STARCH HYDROLYSIS TESTING
Amylase enzyme
Starch
Blue-black color
NEGATIVE for amylase production
Glucose
No color
POSITIVE for amylase production


MICRO & IMMUNO
INDEX

